Idiosyncratic Drug Reaction Risk Checker
This tool helps you understand potential idiosyncratic drug reactions based on the medication you're taking. Remember: this is for educational purposes only and should not replace professional medical advice.
Most people know what a common side effect feels like - a headache after taking a new painkiller, or an upset stomach from antibiotics. These are predictable. They happen to many people, and doctors expect them. But there’s another kind of reaction that no one sees coming. It doesn’t follow the rules. It doesn’t care how much you took. It strikes one person out of 10,000 - sometimes one in 100,000 - and when it does, it can be life-threatening. These are idiosyncratic drug reactions.
What Makes a Drug Reaction Idiosyncratic?
Idiosyncratic drug reactions, or IDRs, are not caused by the drug’s intended action. They’re not dose-dependent. You can take the same dose as your neighbor, and they’re fine while you end up in the hospital. That’s the definition of idiosyncratic: random, unusual, personal. These reactions are also called Type B adverse drug reactions, and they make up only about 13-15% of all drug side effects. But here’s the catch: they’re responsible for nearly half of all drugs pulled off the market.
Think about it - drugs go through years of testing. Thousands of people take them in clinical trials. Still, these reactions slip through. Why? Because they’re not about the drug alone. They’re about you - your genes, your immune system, your body’s unique chemistry. Something in your system turns a harmless molecule into a trigger for chaos.
When Do These Reactions Happen?
One of the most confusing things about IDRs is the delay. You start a new medication. You feel fine. Then, two weeks later, you get a rash. Or three weeks later, your skin starts peeling. Or four weeks in, your liver enzymes spike and you’re jaundiced. That’s not a mistake. That’s normal for IDRs.
According to data from the Drug-Induced Liver Injury Network, most severe reactions appear between one and eight weeks after starting the drug. This delay is why so many get misdiagnosed. A fever, fatigue, and rash? Doctors think it’s the flu. A swollen liver? Maybe hepatitis. It takes time - and sometimes a lot of frustration - to connect the dots back to the medication.
Common Types of Idiosyncratic Reactions
Not all IDRs look the same. The two most common and dangerous types are:
- Idiosyncratic Drug-Induced Liver Injury (IDILI): This is the #1 cause of sudden liver failure from medications. It shows up in two main ways - either the liver cells get damaged (hepatocellular injury), or bile flow gets blocked (cholestatic injury). About 45-50% of all serious drug-related liver damage is idiosyncratic. Drugs like acetaminophen (in high doses), statins, and some antibiotics can cause it. Troglitazone, a diabetes drug pulled in 2000, killed patients through this exact mechanism.
- Severe Cutaneous Adverse Reactions (SCARs): These are skin and mucous membrane disasters. They include Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and DRESS (Drug Reaction with Eosinophilia and Systemic Symptoms). TEN is terrifying - up to 30% of your skin can detach. Mortality for TEN is 25-35%. Carbamazepine, allopurinol, and sulfonamides are common culprits.
Other rarer forms include blood disorders, kidney damage, and lung inflammation. The pattern? Always unexpected. Always severe. Always hard to predict.
Why Do Only Some People Get Them?
This is where science gets fascinating - and frustrating.
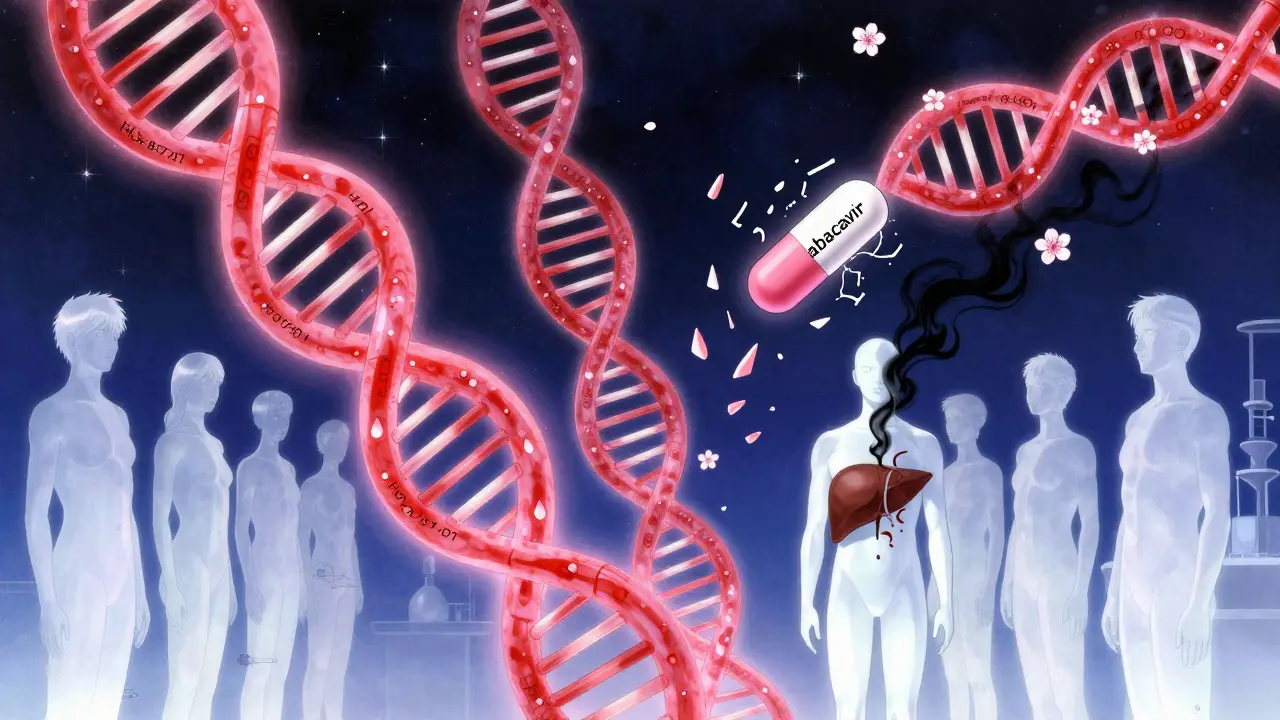
For most drugs, we have no idea why one person reacts and another doesn’t. But there are a few exceptions where genetics gives us a warning. The most famous example is HLA-B*57:01. If you carry this gene variant, taking the HIV drug abacavir can trigger a life-threatening allergic reaction. Test for it first, and the risk drops to near zero. That’s why all HIV patients now get tested before starting abacavir.
Another example is HLA-B*15:02. People with this gene, especially in Southeast Asia, are at extreme risk for SJS/TEN when taking carbamazepine. Screening is now standard in those regions.
But here’s the problem: those are the only two cases where we’ve cracked the code. For 92% of IDRs, there’s no genetic test. No blood marker. No way to know until it’s too late. That’s why the FDA says most serious reactions happen without any warning signs.
How Do Doctors Diagnose Them?
There’s no single test for IDRs. Diagnosis is detective work. Doctors use tools like the RUCAM scale for liver injury and the ALDEN score for skin reactions. Both look at timing, symptoms, and whether other causes (like infection or another drug) could explain it.
The gold standard is the dechallenge: stop the drug. If symptoms improve within days or weeks, that’s a strong clue. Rechallenge - giving the drug back to see if the reaction returns - is rarely done. It’s too dangerous.
Some specialized centers use lymphocyte transformation tests, which check if your immune cells react to the drug in a lab. But these tests aren’t widely available and only work for a few types of reactions.
And here’s the hard truth: many doctors don’t know how to spot these reactions. A 2022 survey found that 65% of patients with IDRs were initially misdiagnosed. One patient described being told she had “just a virus” for two weeks while her skin was literally falling off.
What Happens After a Reaction?
Surviving an IDR doesn’t mean you’re out of the woods. About 28% of people with drug-induced liver injury develop chronic liver problems. Some need lifelong monitoring. Others need transplants.
Financially, it’s brutal. The average cost of a severe IDR event is $47,500 - not including lost wages or long-term care. Many patients lose jobs. Insurance battles drag on. The emotional toll is just as heavy. On patient forums, the most common complaints are: “I felt dismissed,” “No one took me seriously,” and “I was blamed for being ‘sensitive.’”
But there’s hope. Specialized clinics like the Mayo Clinic Drug Safety Clinic have cut diagnosis time from 14 days to under 5 days by using standardized protocols. Their patient satisfaction rate? 92%.

What’s Being Done to Prevent Them?
The pharmaceutical industry is spending billions trying to stop IDRs before they happen. In 2005, only 35% of drug companies screened for reactive metabolites - the toxic byproducts that often trigger immune reactions. Today, it’s 92%. Pfizer and others now use strict thresholds: if a drug produces more than 50 picomoles of reactive metabolites per milligram of protein, it’s flagged for redesign.
Regulators are catching up too. The FDA now requires detailed metabolite testing for any drug that exposes patients to more than 10% of the parent compound’s levels. The EMA requires immune monitoring for all new kinase inhibitors. In 2023, the FDA approved the first predictive test for pazopanib liver toxicity - a breakthrough.
Big projects are underway. The NIH is investing $47.5 million in the Drug-Induced Injury Network. The European Commission is funding ADRomics, a $32.7 million project using AI and genomics to predict reactions by 2027. McKinsey predicts a 40% drop in IDR-related drug failures by 2030.
But experts like Dr. Jack Uetrecht warn: “We’ll never eliminate these reactions. The immune system is too complex.” The goal isn’t perfection. It’s prevention. Better screening. Earlier detection. Less suffering.
What Should You Do?
If you’re starting a new medication:
- Know the common side effects - but also know the red flags: unexplained rash, fever, dark urine, yellow eyes, severe fatigue, swollen lymph nodes.
- Track when symptoms start. If they appear after 1-8 weeks, question the drug.
- Don’t ignore symptoms just because “it’s rare.” Rare doesn’t mean impossible.
- Ask your doctor: “Is there a genetic test for this drug?” Especially if you’re of Asian descent and taking carbamazepine, or have HIV and might get abacavir.
- Keep a medication log. Include dates, doses, and any new symptoms. Bring it to every appointment.
If you’ve had a reaction before, make sure every provider knows. Put it in your medical record. Wear a medical alert bracelet if it was severe.
Most importantly: if something feels wrong, speak up. Don’t wait for your doctor to notice. You’re the expert on your own body.
Where to Find Reliable Information
Don’t rely on random websites. Use trusted resources:
- LiverTox (from the National Institutes of Health): Updated weekly. Lists drugs linked to liver injury with detailed clinical profiles.
- RegiSCAR: A European registry for SCARs with diagnostic criteria and case reports.
- Liverpool Drug Interaction Group: A free database used by over 4 million people annually for checking drug interactions and risks.
These aren’t just academic tools. They’re used by doctors every day to save lives.